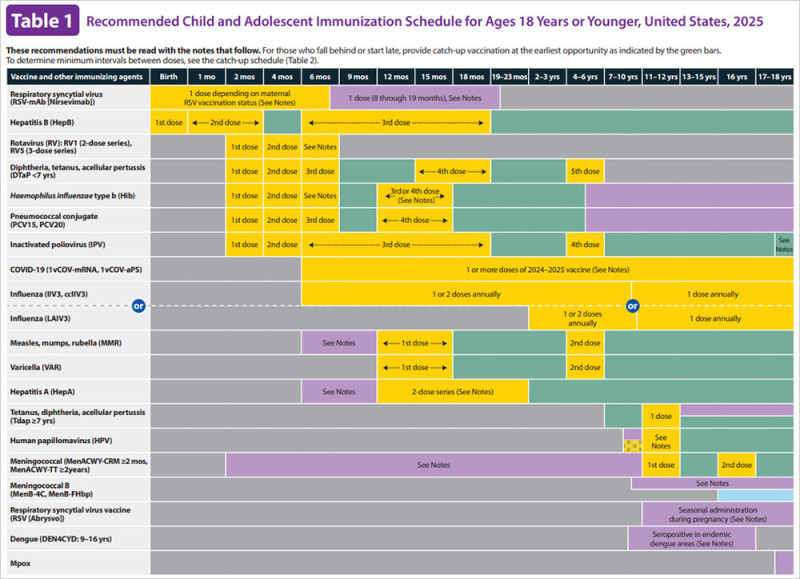
The hepatitis B vaccine is a critical component of public health strategies worldwide, playing a pivotal role in preventing the spread of hepatitis B, a viral infection that can lead to serious liver complications, including liver cancer and cirrhosis. The CDC (Centers for Disease Control and Prevention) and the ACIP (Advisory Committee on Immunization Practices) have established comprehensive guidelines to ensure effective vaccination across various age groups. This article delves into the current CDC hepatitis B vaccine schedule and its significance in 2025, highlighting the key recommendations and updates that have shaped the immunization landscape.
The CDC and ACIP have made significant strides in refining their recommendations to accommodate the evolving public health needs. The Advisory Committee on Immunization Practices (ACIP) recommends hepatitis B (HepB) vaccination for all infants at birth, unvaccinated children under 19, and adults aged 19-59, as well as those 60 and older with risk factors or seeking protection. This broadened recommendation underscores the universal importance of hepatitis B vaccination, ensuring that individuals across all age groups are protected against the virus.
For infants, the first dose of the hepatitis B vaccine is typically administered at birth, with subsequent doses following a recommended schedule. However, recent updates have altered the birth dose recommendation. The CDC's vaccine advisory panel voted to change the recommendation for when children should receive their first dose, indicating a shift towards a more nuanced approach to vaccination timing. This decision reflects a growing emphasis on shared clinical decision-making, where parents and healthcare providers consider the benefits, risks, and individual infection risks before deciding on the vaccination timeline.
For individuals of all ages, the hepatitis B vaccine is typically administered in a series of three or four doses, depending on the specific product and age group. The three-dose series is commonly used, with the second dose given 1-2 months after the first and the third dose 2-4 months after the second. The four-dose series is less common, primarily used in infants, and follows a slightly different interval between doses .
In 2025, the CDC released updated guidelines that emphasize the importance of completing the vaccine series with age-appropriate products. This flexibility ensures that individuals can continue their vaccination series even if they switch between different vaccine brands or products. For instance, if a patient is mid-series after receiving a PreHevbrioTM vaccine, they can complete the series with any other age-appropriate product. This adaptability is particularly relevant as new vaccines are introduced or existing ones are withdrawn from the market.
The CDC's hepatitis B vaccine schedule is a dynamic and evolving framework, reflecting ongoing research, public health priorities, and the development of new vaccines. As of December 2025, the recommendations continue to emphasize the importance of universal vaccination and the need for individualized decision-making. The CDC's efforts to strengthen its recommendations are a testament to its commitment to public health, ensuring that all individuals have access to effective protection against hepatitis B.